Link: Articles on PubMed
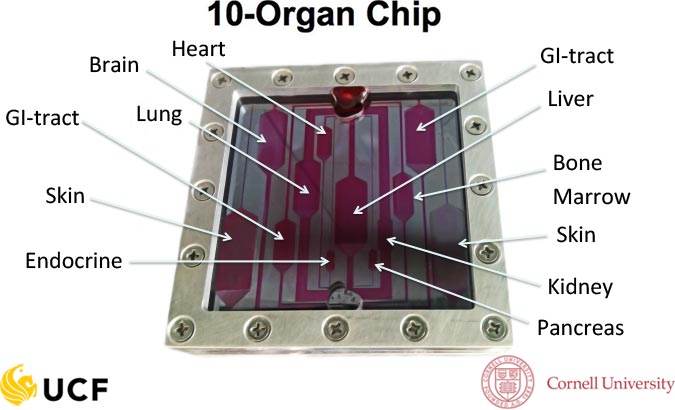
As part of an initiative from the National Center for Advancing Translational Sciences (NCATS), the Hybrid Systems Lab is collaborating with Michael Shuler and his team at Cornell University to produce a physiologically accurate model of the human body incorporating 10 organ systems interconnected using a microfluidic platform. It is hoped that this “Body-On-A-Chip” model will provide the means to generate highly predictive and in depth analyses of novel therapeutic candidates during preclinical development which far exceed what is possible currently using more basic, single cell type assays. The successful development of such advanced human in vitro models may also pave the way for the reduction and eventual replacement of animals in preclinical drug development which are costly, time-consuming and often poor predictors of human responses to drugs[16, 17, 18].
Current work on this project has focused on the development of microphysiological modules to accurately model the functionality of the nervous, circulatory and gastrointestinal tract systems in vitro. We are now progressing toward the integration of such systems into a single microfluidic platform utilizing a serum-free blood surrogate, as well as the development and incorporation of additional organ systems including skin and liver.
References
[1] Esch MB, Smith AS, Prot JM, Oleaga C, Hickman JJ, Shuler ML. How multi-organ microdevices can help foster drug development. Advanced drug delivery reviews. 2014.
[2] Smith AST, Long CJ, Pirozzi K, Hickman JJ. A functional system for high-content screening of neuromuscular junctions in vitro. Technology. 2013;1:37-48.
[3] Sung JH, Esch MB, Prot JM, Long CJ, Smith A, Hickman JJ, et al. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab on a chip. 2013;13:1201-12.
[4] Smith AS, Long CJ, Berry BJ, McAleer C, Stancescu M, Molnar P, et al. Microphysiological systems and low-cost microfluidic platform with analytics. Stem cell research & therapy. 2013;4 Suppl 1:S9.
[5] Guo X, Ayala JE, Gonzalez M, Stancescu M, Lambert S, Hickman JJ. Tissue engineering the monosynaptic circuit of the stretch reflex arc with co-culture of embryonic motoneurons and proprioceptive sensory neurons. Biomaterials. 2012;33:5723-31.
[6] Rumsey JW, Das M, Bhalkikar A, Stancescu M, Hickman JJ. Tissue engineering the mechanosensory circuit of the stretch reflex arc: Sensory neuron innervation of intrafusal muscle fibers. Biomaterials. 2010;31:8218-27.
[7] Wilson K, Das M, Wahl KJ, Colton RJ, Hickman JJ. Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLoS ONE. 2010;5.
[8] Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31:4880-8.
[9] Das M, Rumsey JW, Bhargava N, Gregory C, Riedel L, Kang JF, et al. Developing a novel serum-free cell culture model of skeletal muscle differentiation by systematically studying the role of different growth factors in myotube formation. In Vitro Cell Dev Biol Anim. 2009;45:378-87.
[10] Wilson K, Molnar P, Hickman JJ. Integration of functional myotubes with a Bio-MEMS device for non-invasive interrogation. Lab on a chip. 2007;7:920-2.
[11] Das M, Gregory CA, Molnar P, Riedel LM, Wilson K, Hickman JJ. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27:4374-80.
[12] Das M, Molnar P, Devaraj H, Poeta M, Hickman JJ. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnology progress. 2003;19:1756-61.
[13] Stenger DA, Hickman JJ, Bateman KE, Ravenscroft MS, Ma W, Pancrazio JJ, et al. Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. Journal of neuroscience methods. 1998;82:167-73.
[14] Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, et al. Developmental Neurobiology Implications from Fabrication and Analysis of Hippocampal Neuronal Networks on Patterned Silane-Modified Surfaces. Journal of the American Chemical Society. 1998;120:12169-77.
[15] Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. Journal of neuroscience methods. 1995;62:111-9.
[16] Hickman JJ, Bhatia SK, Quong JN, Shoen P, Stenger DA, Pike CJ, et al. Rational pattern design for in vitro cellular networks using surface photochemistry. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 1994;12:607-16.
[17] Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Research. 1993;630:136-47.
[18] Stenger DA, Georger JH, Dulcey CS, Hickman JJ, Rudolph AS, Nielsen TB, et al. Coplanar molecular assemblies of amino- and perfluorinated alkylsilanes: characterization and geometric definition of mammalian cell adhesion and growth. Journal of the American Chemical Society. 1992;114:8435-42.