Photochromism is a non-destructive process involving rearrangement of chemical bonds. Dithienyl perfluorocyclopentenes are an important class of thermally irreversible (P-type) photochromic compounds (Fig.1). They undergo light-induced reversible transition from open to closed ring isomers accompanied by the change in color and other properties. In order to be useful in design of various optoelectronic devices such as optical memory, optical switching, displays and nonlinear optics, the photochromic material has to satisfy certain requirements, such as 1) thermal stability of both isomers; 2) fatigue resistance; 3) efficient photochromic reactivity: high sensitivity and rapid response; 4) high solubility in polymer matrices; 5) nondestructive readout capability; 6) sensitivity at diode laser wave lengths [1].
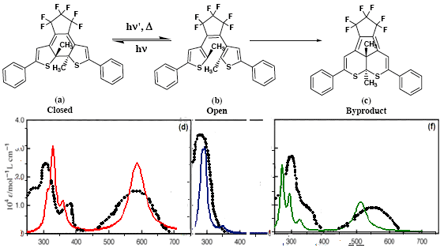
During the photochromic rearrangement, undesirable chemical reactions may also occur. This limits the number of cycles of photochromic reactions and contributes to photochemical fatigue. If the formation mechanism of undesirable byproduct is known, its kinetics and therefore fatigue resistance can be predicted. Fig. 1 depicts the closed, open and byproduct isomeric forms of 1,2-bis(2-methyl-5-phenyl-3-thienyl)perfluorocyclopentene (PFC) synthesized by Irie and coworkers [2]. The mechanism for the formation of the byproduct is not well understood. The thermal cycloreversion in this system has activation barrier of 33 kcal/mol and absorption spectra are recorded for all the three isomers. The fatigue resistance is reported as 73% decrease in the absorbance of the open form after 850 cycles.
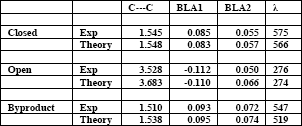
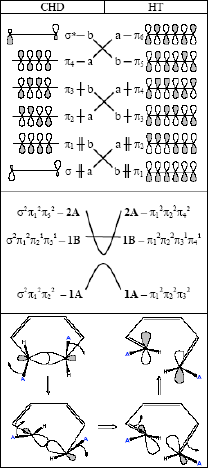
In this contribution we apply Density Functional Theory (DFT) to predict the equilibrium geometry (Table 1) and absorption spectra (Fig. 1) for all three isomers of PFC. We also study the activation barrier for thermal cycloreversion and the mechanism for the byproduct formation. Thermal cycloreversion process occurs through symmetry forbidden conrotatory electrocyclic mechanism, with transition state of strong diradical character as sketched on the correlation diagrams (Fig. 2). Following the photoactivation, molecular system arrives at the ground state potential surface close to the conical intersection of well documented structure. On this surface the system can evolve to the closed form (1-6 ring closure), or to the metastable diradical intermediate (1-5 ring closure), then rearrange to the byproduct.
The quantitative agreement between the experimental and the theoretical values suggests that DFT methods could be successfully employed in the prediction of three out of six properties essential for design of photoswitching and data storage applications: thermal stability, fatigue resistance and absorption wavelength.
References
[1] M. Irie, Chem. Rev. 100, 1685 (2000).
[2] M. Irie, T. Lifka, K. Uchida, S. Kobatake, Y. Shindo, Chem. Commun., 747 (1999).