Diarylethenes are organic molecules (Fig.1), capable to undergo transition from the open to the closed ring isomer under illumination (photocyclization). This transition is accompanied by the light-induced change in color (photochromism), and may be used for optical data storage and ultrafast photoswitching applications with individually addressable 3-dimensional arrays of bits. Many hundreds of layers beneath optical disk surface may be used, increasing storage capacity two orders of magnitude. Application of diarylethenes in for storage and switching was extensively studied for the past decade [1]. In addition, two-photon absorption (2PA) of light was suggested to reduce the parasite cross-talk between these recorded layers [2]. 2PA is a nonlinear optical process in which chromophores that would normally be excited by a single-photon of shorter wavelength are excited by two photons of longer wavelengths. The probability of 2PA is quadratically dependent on the intensity of the excitation light, resulting in greater 3D spatial selectivity through the use of a tightly focused laser beam. This contribution addresses the challenges of combining 2PA with photochromism in one molecule and proposes a computational approach to rational design of these molecules, based on Time-Dependent Density Functional Theory (TD-DFT).
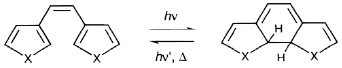
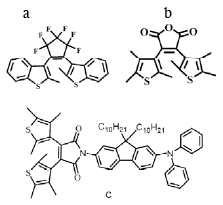
In effort to design 2PA-initiated photoswitch, the molecule c (Fig.2) was synthesized in the Department of Chemistry at UCF. It contains diarylethene phochromic moiety and fluorene- based 2PA chromophore substituent. However, this substitution led to the loss of photochromic activity.
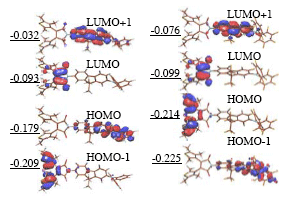
Analysis of the Kohn-Sham orbitals (Fig.3) reveals that the HOMO-1 to LUMO excitation is localized on diarylethene fragment and leads to photocyclization. However, the HOMO is localized on the donor substituent and gives rise to an excited state below the photoreactive one. According to Kasha’s rule 2PA state then decays to the photostable HOMO-LUMO state. As a result, the molecule c is not functioning as designed. To make the molecular switch active in two-photon regime we suggest stabilizing the highest occupied orbital (HOMO) below HOMO-1, which can be accomplished by fluorination of the fluorene fragment.
We applied Coupled Electronic Ocsillator formalism [3], combined with TD-DFT to predict 2PA profiles of cF. The method had recently shown superior accuracy in predictions of 2PA cross-sections [4]. We conclude that fluorination does not reduce 2PA cross-sections significantly. In order to accurately describe section of the potential energy surface (PES) along symmetry-forbidden pericyclic reaction pathway passing through the transition state of a strong biradical character, the new exchange-correlation functional was developed. This functional correctly reproduces symmetry breaking in model systems as compared to highly correlated ab initio results, and will be described in details elsewhere. We used the new functional in conjunction with unrestricted Kohn-Sham formalism to construct the PES of the ground state for conrotatory cyclization. The vertical excitation energies were obtained with restricted TD-DFT formalism and conventional B3LYP exchange-correlation functional. They were used to build PES of the excited state. For c this PES does have potential barrier between Franck-Condon region and the pericyclic minimum, while for cF it does not, in agreement with simple orbital predictions.
References
[1] M. Irie, Chemical Reviews2000, 100, 1685.
[2] D. A. Parthenopoulos, P. M. Rentzepis, Science1989, 245, 843.
[3] S. Tretiak, S. Mukamel, Chemical Reviews2002, 102, 3171.
[4] A. M. Masunov, S. Tretiak, Journal of Physical Chemistry B2004, 108, 899.